When Can I Expect to Get My COVID-19 Vaccine?
Keeping up with COVID-19 news, including the new vaccines’ efficacy, availability, and eligibility, is without a doubt challenging.
If you are an immunocompromised patient, the information provided by media outlets is not only confusing, it often seems to ignore your condition.
Some of the top concerns are related to the following topics:
- How far along are the different vaccines in the approval process
- The distribution process
- Who are the priority populations
- When is the vaccine available to chronic disease patients
This article will try to clarify some of these concerns and help you better understand how the new COVID-19 vaccines work.
How Are COVID-19 Vaccines Different?
The innovative messenger RNA (mRNA) vaccines work differently than traditional vaccines.
Instead of using a weakened or inactivated virus to produce an immune response, the COVID-19 mRNA vaccine provides our cells instructions on how to make a unique spike protein. This spike protein is very similar to the one found on the surface of the coronavirus that causes COVID-19.
When the cells make the protein and display the spike on their surfaces, it triggers an immune response. The immune system will then create antibodies to attack the foreign invader, in this case, the virus responsible for COVID-19.
How Far Along Are the Different Vaccines in the Approval Process
Since the beginning of the pandemic, the research community, as global effort, worked to develop vaccines to protect us from COVID-19. These remarkable efforts resulted in record time vaccine development.
In the United States, the two vaccines that received emergency authorization from the Food and Drug Administration (FDA) were:
Additionally, there are three large-scale (Phase 3) COVID-19 vaccine clinical trials in progress or planned in the United States:
- AstraZeneca’s
- Novavax’s
- Janssen’s

Vaccine Distribution and Allocation
The FDA issued its first emergency use authorization for the Pfizer-BioNTech COVID-19 vaccine on December 11th and gave its second emergency use authorization for Moderna’s a week later.
Vaccine deliveries began in mid-December and continue to arrive at sites across the country. Since supplies are limited, each state and its jurisdiction receive a specific amount of COVID-19 vaccine doses. Each state and jurisdiction is then responsible for the distribution and administration of the vaccines.
Priority Populations
The Centers for Disease Control and Prevention (CDC) made recommendations on who should be vaccinated first while supplies are limited.
The recommendations aimed to achieve the following goals:
- Decrease death and severe disease as much as possible
- Preserve the functioning of society
- Reduce the extra burden the disease is having on groups already facing disparities
CDC’s Vaccine Rollout Recommendations
Phase 1a – Healthcare personnel and long-term care facility residents
Phase 1b – Frontline essential workers and seniors 75 years and older
Phase 1c – Individuals aged 65—74 years of age. People aged 16—64 years of age with underlying medical conditions that could increase the risk of severe disease. Other essential workers.
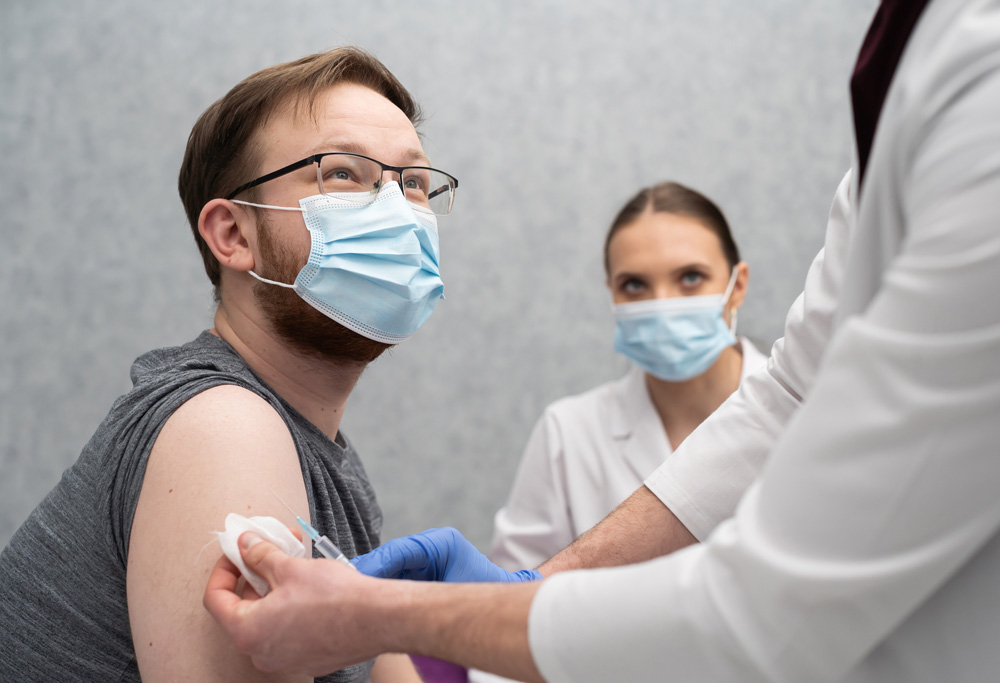
When Will the Vaccine be Available to Chronic Disease Patients?
The FDA considers the current COVID-19 vaccines to be safe for immunocompromised patients. There is a concern of a potential decreased response to the vaccine due to drug interaction.
As a result of this, it’s vital for you to speak to your doctor and follow their recommendations before getting vaccinated.
Although the CDC makes recommendations on which groups need to be prioritized, each state may have its own plan on who is eligible to be vaccinated first.
For this reason, Altus Biologics advises you to first speak with your physician then check your state and local health department to find out when the vaccine will be made available for you. The CDC has made it easy for you to get the latest information from health departments near you. You can access this resource by clicking here.





